Copper Chloride Dissolved In Water . in this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is dissolved in water. Besides this, it is a. So for each mole of copper chloride, full dissociation will give you. on dilution the colour changes to green and then blue because of successive replacement of chloride ions by water. It is also soluble in alcohol. when cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2] is produced. if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. to tell if cucl2 (copper (ii) chloride) forms an acidic, basic (alkaline), or neutral solution we can use these three. copper (ii) chloride can oxidize and dissolve aluminium, owing to the formation of the tetrachlorocuprate ion. The compound is soluble in water, and the solubility increases with temperature.
from www.numerade.com
The compound is soluble in water, and the solubility increases with temperature. in this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is dissolved in water. copper (ii) chloride can oxidize and dissolve aluminium, owing to the formation of the tetrachlorocuprate ion. when cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2] is produced. It is also soluble in alcohol. So for each mole of copper chloride, full dissociation will give you. on dilution the colour changes to green and then blue because of successive replacement of chloride ions by water. to tell if cucl2 (copper (ii) chloride) forms an acidic, basic (alkaline), or neutral solution we can use these three. if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. Besides this, it is a.
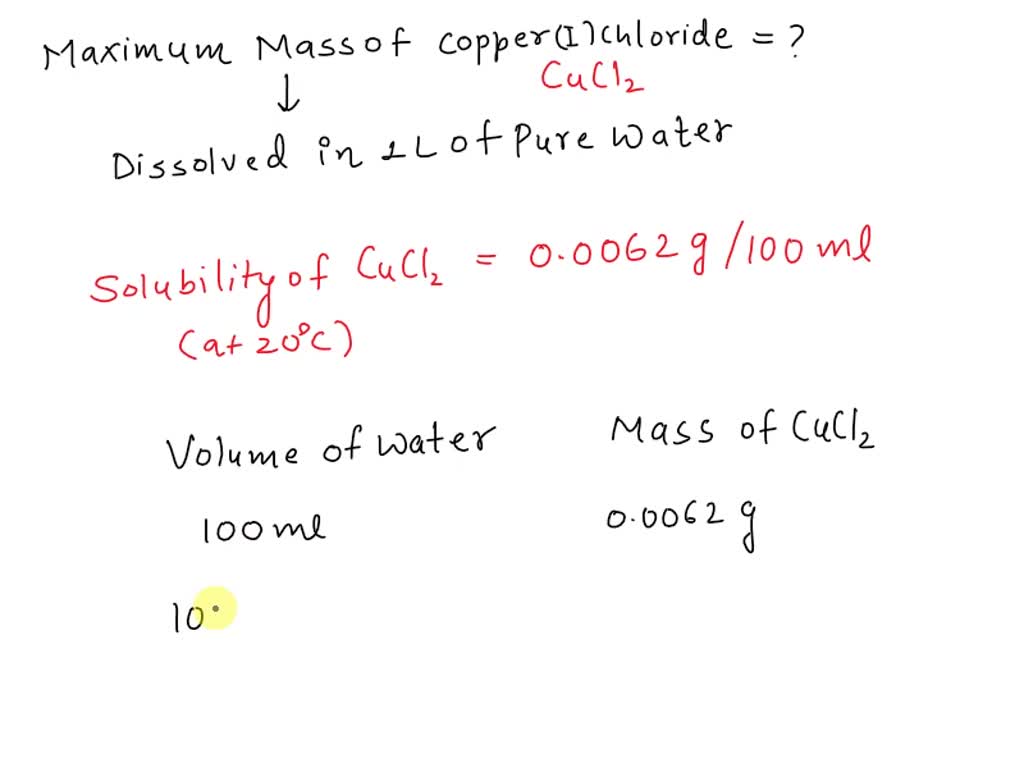
SOLVED What is the maximum mass of copper I Chloride that can be
Copper Chloride Dissolved In Water Besides this, it is a. if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. The compound is soluble in water, and the solubility increases with temperature. Besides this, it is a. So for each mole of copper chloride, full dissociation will give you. when cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2] is produced. copper (ii) chloride can oxidize and dissolve aluminium, owing to the formation of the tetrachlorocuprate ion. It is also soluble in alcohol. to tell if cucl2 (copper (ii) chloride) forms an acidic, basic (alkaline), or neutral solution we can use these three. in this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is dissolved in water. on dilution the colour changes to green and then blue because of successive replacement of chloride ions by water.
From www.youtube.com
How to write the equation for CuO + H2O Copper (II) oxide + Water Copper Chloride Dissolved In Water to tell if cucl2 (copper (ii) chloride) forms an acidic, basic (alkaline), or neutral solution we can use these three. It is also soluble in alcohol. So for each mole of copper chloride, full dissociation will give you. in this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is dissolved in. Copper Chloride Dissolved In Water.
From www.scribd.com
Electrolysis of concentrated aqueous copper chloride1 PDF Copper Chloride Dissolved In Water to tell if cucl2 (copper (ii) chloride) forms an acidic, basic (alkaline), or neutral solution we can use these three. if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. The compound is soluble in water, and the solubility increases with temperature. when cucl 2 is dissolved in h 2 o, a beautiful green. Copper Chloride Dissolved In Water.
From pixels.com
Copper (ii) Chloride Solution Photograph by Martyn F. Chillmaid/science Copper Chloride Dissolved In Water when cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2] is produced. to tell if cucl2 (copper (ii) chloride) forms an acidic, basic (alkaline), or neutral solution we can use these three. copper (ii) chloride can oxidize and dissolve aluminium, owing to the. Copper Chloride Dissolved In Water.
From www.numerade.com
SOLVEDA concentrated aqueous copper(II) chloride solution is bright Copper Chloride Dissolved In Water on dilution the colour changes to green and then blue because of successive replacement of chloride ions by water. in this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is dissolved in water. Besides this, it is a. when cucl 2 is dissolved in h 2 o, a beautiful green. Copper Chloride Dissolved In Water.
From www.researchgate.net
Partial clarification of copper chloride. Top spectra is copper Copper Chloride Dissolved In Water when cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2] is produced. So for each mole of copper chloride, full dissociation will give you. on dilution the colour changes to green and then blue because of successive replacement of chloride ions by water. The. Copper Chloride Dissolved In Water.
From www.researchgate.net
Water vapor absorption curves of the three (A) copper chloride, (B Copper Chloride Dissolved In Water Besides this, it is a. copper (ii) chloride can oxidize and dissolve aluminium, owing to the formation of the tetrachlorocuprate ion. on dilution the colour changes to green and then blue because of successive replacement of chloride ions by water. The compound is soluble in water, and the solubility increases with temperature. So for each mole of copper. Copper Chloride Dissolved In Water.
From www.youtube.com
Synthesis of Copper Chloride YouTube Copper Chloride Dissolved In Water on dilution the colour changes to green and then blue because of successive replacement of chloride ions by water. Besides this, it is a. if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. in this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is dissolved in. Copper Chloride Dissolved In Water.
From www.coursehero.com
[Solved] During part B of the lab, the copper chloride anhydrate was Copper Chloride Dissolved In Water if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. Besides this, it is a. on dilution the colour changes to green and then blue because of successive replacement of chloride ions by water. So for each mole of copper chloride, full dissociation will give you. in this video we will describe the equation. Copper Chloride Dissolved In Water.
From www.pinterest.com
Electrolysing copper chloride solution to show its made of copper and Copper Chloride Dissolved In Water So for each mole of copper chloride, full dissociation will give you. if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. The compound is soluble in water, and the solubility increases with temperature. on dilution the colour changes to green and then blue because of successive replacement of chloride ions by water. when. Copper Chloride Dissolved In Water.
From www.youtube.com
Classic Reaction Aluminum and Copper Chloride HausLab Chemistry Copper Chloride Dissolved In Water if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. It is also soluble in alcohol. So for each mole of copper chloride, full dissociation will give you. Besides this, it is a. when cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2. Copper Chloride Dissolved In Water.
From webmis.highland.cc.il.us
Aqueous Solutions Copper Chloride Dissolved In Water when cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2] is produced. It is also soluble in alcohol. So for each mole of copper chloride, full dissociation will give you. to tell if cucl2 (copper (ii) chloride) forms an acidic, basic (alkaline), or neutral. Copper Chloride Dissolved In Water.
From mmerevise.co.uk
Reactions of Acids Worksheets and Revision MME Copper Chloride Dissolved In Water if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. It is also soluble in alcohol. Besides this, it is a. when cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2] is produced. on dilution the colour changes to green. Copper Chloride Dissolved In Water.
From chemistry.stackexchange.com
experimental chemistry Reaction between copper (II)chloride and Copper Chloride Dissolved In Water in this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is dissolved in water. Besides this, it is a. if you assume a molecule $\ce{cucl2}$ there is one copper and two chlorine. It is also soluble in alcohol. on dilution the colour changes to green and then blue because of. Copper Chloride Dissolved In Water.
From maverickkruwfowler.blogspot.com
Colour of Copper Chloride MaverickkruwFowler Copper Chloride Dissolved In Water So for each mole of copper chloride, full dissociation will give you. The compound is soluble in water, and the solubility increases with temperature. when cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2] is produced. copper (ii) chloride can oxidize and dissolve aluminium,. Copper Chloride Dissolved In Water.
From chemistrycalc.com
Copper I Chloride Formula Copper Chloride Dissolved In Water on dilution the colour changes to green and then blue because of successive replacement of chloride ions by water. It is also soluble in alcohol. copper (ii) chloride can oxidize and dissolve aluminium, owing to the formation of the tetrachlorocuprate ion. when cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to. Copper Chloride Dissolved In Water.
From www.numerade.com
SOLVED What is the maximum mass of copper I Chloride that can be Copper Chloride Dissolved In Water The compound is soluble in water, and the solubility increases with temperature. Besides this, it is a. in this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is dissolved in water. It is also soluble in alcohol. when cucl 2 is dissolved in h 2 o, a beautiful green color due. Copper Chloride Dissolved In Water.
From www.coursehero.com
[Solved] Sample A contains 0.715 g of copper chloride dissolved in Copper Chloride Dissolved In Water on dilution the colour changes to green and then blue because of successive replacement of chloride ions by water. to tell if cucl2 (copper (ii) chloride) forms an acidic, basic (alkaline), or neutral solution we can use these three. The compound is soluble in water, and the solubility increases with temperature. if you assume a molecule $\ce{cucl2}$. Copper Chloride Dissolved In Water.
From www.animalia-life.club
Copper Chloride Copper Chloride Dissolved In Water It is also soluble in alcohol. Besides this, it is a. in this video we will describe the equation cucl2 + h2o and write what happens when cucl2 is dissolved in water. So for each mole of copper chloride, full dissociation will give you. when cucl 2 is dissolved in h 2 o, a beautiful green color due. Copper Chloride Dissolved In Water.